Does Volume Depend on Time?
A weird kinetics problem where the solution volume keeps shrinking, so the concentration first drops and then suddenly starts going up
Problem
A first-order reaction $(A \rightarrow products)$ takes place in an aqueous solution. At the same time, while $A$ is converting into products, the water in the solution is evaporating. The decrease of the solution volume is described by the equation: $V(t) = V(t=0) - \alpha t$. Does the concentration of reagent A increase or decrease in the solution over time?
Solution
To begin with, we need to understand the idea itself. What does it mean for a concentration to increase or decrease over time? This is simply a question about determining the monotonic behavior of a function: $C=f(t)=C(t)$. There are multiple ways to approach this.
For example, suppose we want to check whether the concentration increases. Then we might compare values such as $C(t+1) > C(t)$: if at a later time the concentration is higher, the function is increasing. However, this approach is not always reliable and can fail to give the correct result.
A much simpler and more powerful method is to examine the sign of the first derivative. Why does this work? Let’s recall the definition of a derivative: $$\frac{dC(t)}{dt}=\lim_{\Delta t\to \infty}\frac{C(t+\Delta t)-C(t)}{\Delta t}$$
Oops, right — earlier we only looked at the case where the function is increasing, meaning $C(t + \Delta t) > C(t)$. Here we have the same type of inequality, and it’s true when $\Delta t > 0$. So if the derivative is positive, the concentration is growing. You can show the same thing for a decreasing function too (just check the case $\Delta t < 0$ yourself). Okay, now let’s actually get back to the problem. We need to find the explicit form of the concentration $C(t)$. We already know the volume $V(t)$, but we don’t know the amount of substance $n(t)$ yet. So we’ll use the law of mass action. It gives us: $$r(t)=-\frac{1}{V(t)}\frac{dn(t)}{dt}=\frac{1}{V(t)}kn(t)$$
But wait a second. Since the volume depends on time, we can’t just hide it inside the derivative. Instead, let’s multiply both sides of the equation by $V(t)$: $$-\frac{dn(t)}{dt}=k n(t)$$ This is the usual differential equation for a first-order reaction. It’s really easy to integrate: $$\int^{n(t)}_{n(0)}\frac{dn}{n}=\int^{n(t)}_{n(0)}d(\ln{n})=-k\int^{t}_{0}dt$$ And after integrating, we get the classic exponential decay: $$n(t)=n(0)\cdot e^{-kt}$$ Now we finally have a clean expression for the concentration, since $$C(t)=\frac{n(t)}{V(t)}=\frac{n(0)\cdot e^{-kt}}{V(0)-\alpha t}$$ Lets take the derivative: $$\frac{dC(A)}{dt}=\frac{d}{dt}(\frac{n(t=0)\cdot e^{-kt}}{V(t=0)-\alpha t})$$ $$\frac{dC(A)}{dt}=\frac{-k\cdot e^{-kt}\cdot n(t=0)(v(t=0)-\alpha t)+\alpha\cdot e^{-kt}\cdot n(t=0)}{(V(t=0)-\alpha t)^2}$$ The square, the exponential, and the initial number of moles are all positive quantities. Therefore, the sign is determined by only one bracket: $$\alpha kt-kV(t=0)+\alpha\lor 0$$ Let us consider the case of increasing concentration: $$\alpha kt-kV(t=0)+\alpha\ge 0$$ $$t\ge\frac{V(t=0)}{\alpha}-\frac{1}{k}=\frac{kV(t=0)-\alpha}{\alpha k}$$ From this, two situations become clear
1) $\alpha>kV(t=0)$. In this case the fraction is negative, and since time is a non-negative quantity, this inequality will hold for any $t$. Let’s plot the graph for specific values: $C(t) = exp(-t)/(1 - 2t)$

2) $kV(t=0)>\alpha$ In this case the situation is less straightforward. Let’s plote the graph using specific numerical values: $C(t)=exp(-t)/(1-0.5t)$
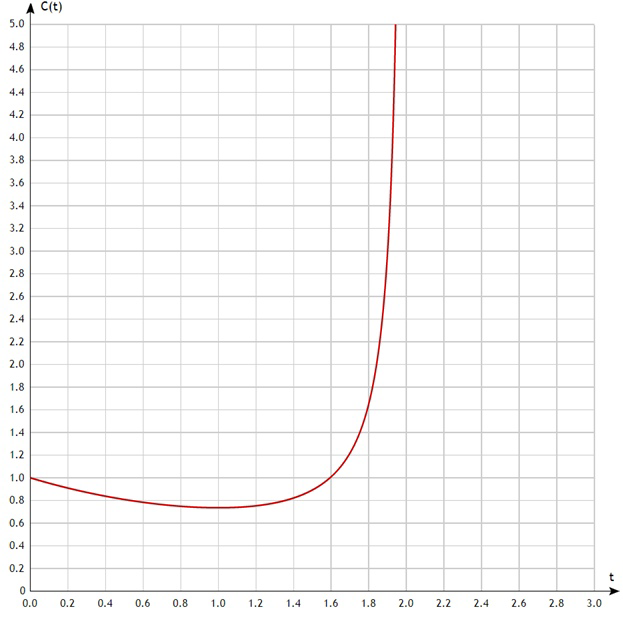
That is, at first the concentration decreases and then, after the inflection point, it begins to increase.
Comments
0Want to join the conversation? Sign in or create an account to share your thoughts.
Be the first to share your thoughts.